
DrugPatentWatch Blog
777 FOLLOWERS
DrugPatentWatch serves leading companies ranging from biopharmaceutical R&D to healthcare delivery and has been cited by CNN, NEJM, Nature Journals, and many other leading publications. Business intelligence on pharmaceutical and biologic drugs - patents, generics, pricing, sales, litigation, and more. Make Better Decisions with DrugPatentWatch.
DrugPatentWatch Blog
1d ago
[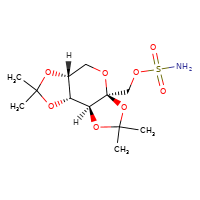](//www.DrugPatentWatch.com/p/preview/generic-api/topiramate?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Topiramate is the generic ingredient in six branded drugs marketed by Upsher Smith Labs, Actavis Labs Fl, Ajanta Pharma Ltd, Alkem Labs Ltd, Dr Reddys, Glenmark Pharms Ltd,…
Source ..read more
DrugPatentWatch Blog
1d ago
Annual Drug Patent Expirations for MEGACE+ES Megace Es is a drug marketed by Endo Pharms Inc and is included in one NDA. There are five patents protecting this drug and…
Source ..read more
DrugPatentWatch Blog
1d ago
This chart shows the pharmaceutical companies with the most syrup dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most syrup dosed drugs…
Source ..read more
DrugPatentWatch Blog
2d ago
Annual Drug Patent Expirations for CONTRAVE Contrave is a drug marketed by Nalpropion and is included in one NDA. It is available from one supplier. There are twenty patents protecting…
Source ..read more
DrugPatentWatch Blog
2d ago
[](//www.DrugPatentWatch.com/p/preview/generic-api/topiramate?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Topiramate is the generic ingredient in six branded drugs marketed by Upsher Smith Labs, Actavis Labs Fl, Ajanta Pharma Ltd, Alkem Labs Ltd, Dr Reddys, Glenmark Pharms Ltd,…
Source ..read more
DrugPatentWatch Blog
2d ago
This chart shows the pharmaceutical companies with the most patents in United Kingdom. Patents must be filed in each country (or, in some cases regional patent office) where patent protection…
Source ..read more
DrugPatentWatch Blog
3d ago
Annual Drug Patent Expirations for XHANCE Xhance is a drug marketed by Optinose Us Inc and is included in one NDA. It is available from one supplier. There are fourteen…
Source ..read more
DrugPatentWatch Blog
3d ago
Annual Drug Patent Expirations for EVRYSDI Evrysdi is a drug marketed by Genentech Inc and is included in one NDA. It is available from one supplier. There are four patents…
Source ..read more
DrugPatentWatch Blog
3d ago
[](//www.DrugPatentWatch.com/p/preview/generic-api/topiramate?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Topiramate is the generic ingredient in six branded drugs marketed by Upsher Smith Labs, Actavis Labs Fl, Ajanta Pharma Ltd, Alkem Labs Ltd, Dr Reddys, Glenmark Pharms Ltd,…
Source ..read more
DrugPatentWatch Blog
4d ago
Annual Drug Patent Expirations for ELIGARD+KIT Eligard Kit is a drug marketed by Tolmar and is included in four NDAs. It is available from one supplier. There is one patent…
Source ..read more